[ad_1]
In 2017, Cindy Sutherland caught a nasty cold she couldn’t shake. After coughing nonstop for weeks, she went to urgent care and got a chest X-ray. When the doctor shared the results with her, he asked, “Have you ever heard of pulmonary fibrosis?” Cindy started to cry because she knew what those words meant. Her grandmother had died of pulmonary fibrosis at the age of 62. Her mother had died of it at 58, and her sister at 48.

The progressive lung disease can be traced to a variety of causes — autoimmune conditions, radiation treatments, specific medications or chemotherapeutic agents, toxins like asbestos or coal dust and environmental factors like mold. Recently, researchers have begun to recognize that a subset of cases can be attributed to genetics. Some patients with pulmonary fibrosis have shortened telomeres, the caps on the ends of chromosomes that protect cells from aging. Identifying these patients is critical because, in their case, certain treatments like immunosuppressive therapies could be dangerous.

“I think it is way more frequent than we realize,” says Sutherland’s pulmonologist, Eva Carmona Porquera, M.D., Ph.D. She is part of a large, multidisciplinary team at Mayo Clinic developing new ways to detect and treat pulmonary fibrosis cases caused by shortened telomeres.
“This is new territory,” says Dr. Carmona Porquera. “The good thing is that at Mayo, we can offer genetic testing and telomere testing to our patients to classify, treat and prepare for complications associated with telomere biology disorders.”
A rare and complex disorder
Telomere biology disorders are rare diseases caused by mutations that accelerate telomere shortening. Patients with these disorders are susceptible to a variety of age-related conditions, including pulmonary fibrosis, bone marrow failure, osteoporosis, cancer and liver fibrosis. Many but not all patients show outward signs of premature aging, such as premature graying of hair or brittle nails.

Hematologist Mrinal Patnaik, M.B.B.S., first encountered these disorders as a medical resident in the early 2000s. A patient in his thirties with completely gray hair had shown up with a malignant tumor at the head and neck cancer clinic where Dr. Patnaik was training. Doctors there did not realize he had a telomere biology disorder. The patient died a month later from complete bone marrow failure.
“That really hurt me deep down,” says Dr. Patnaik. “I felt that there’s so many of these patients who are going to be coming into different clinics and if I didn’t step up and set up a program for them and educate all my colleagues and set up the infrastructure, we’re going to see a lot more patients suffer.”
With the help of the Mayo Clinic Center for Individualized Medicine, Dr. Patnaik established the Telomere Biology Disorders Clinic at the Rochester, Minnesota, campus in 2017. The clinic is multidisciplinary in nature, drawing expertise from hematologists, oncologists, pulmonologists, liver specialists, pharmacists, molecular biologists and genetic counselors. To date, the clinic has seen more than 100 patients with shortened telomeres.
“We are among the largest adult telomere biology disorder clinics in the country,” says Dr. Patnaik, who plans to open similar clinics in Arizona and Florida.
No perfect way
The most frequent manifestation of a telomere biology disorder is pulmonary fibrosis, the scarring of lung tissue that can make it difficult to breathe. As telomeres become shorter and shorter, the chromosomes they are designed to protect can unravel and fray, like a shoelace that has lost its protective cap. Eventually, the telomeres become so short that cells stop dividing — a process known as senescence. Those senescent cells send off a slew of chemicals that act as flares, triggering inflammatory changes that damage and thicken the lungs.
Mayo Clinic researchers hope that by diagnosing and treating the disease earlier, they can improve outcomes for patients.

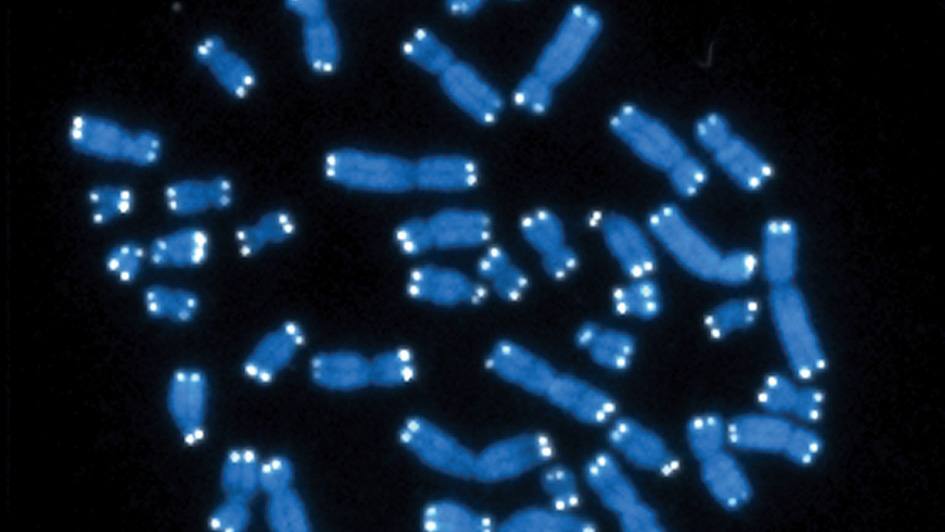
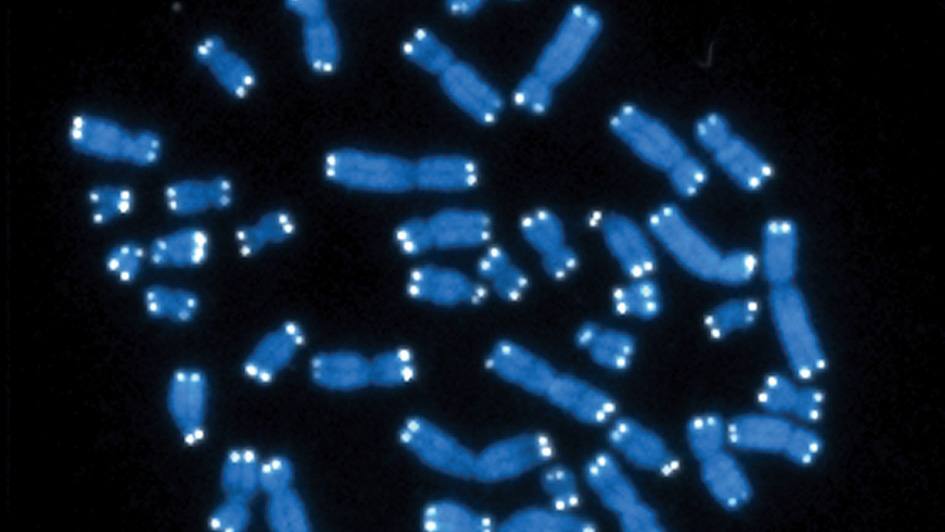
Telomere biology disorders are typically diagnosed by looking for shortened telomeres and testing for genetic mutations. “There are different techniques that have been developed over time to measure telomere length, and each one has pros and cons,” says molecular biologist Alejandro Ferrer, Ph.D. “Basically, there’s no perfect way to do this.”
One of the most common methods for measuring telomeres involves a technique known as Flow FISH, in which each telomere is labeled with a fluorescent marker and then fed into a machine that quantifies the average amount of fluorescence across all the telomeres on all the chromosomes in the cell. Other techniques involve sequencing to count the repetitive run of bases, TTAGGG, that makes up each telomere, again averaging the result.
“However, it’s well-known in the field that it’s not that all telomeres get shorter at the same time; it can be just one or two telomeres falling below a certain threshold that can cause disease,” says Dr. Ferrer. “If you rely on a technique that uses the average, you could miss those cases.”
Dr. Ferrer and collaborators within and outside Mayo Clinic devised a specialized sequencing technique called Telogator to assess the shortest telomere length of each chromosome.
“This test could have diagnostic, prognostic and therapeutic value,” says Dr. Patnaik.
Answers, finally
Even with the limitations of the current tests, the Telomere Biology Clinic is making a positive impact on patients. Sutherland first visited the clinic in 2020 and learned that, on average, her telomere lengths fell below the first percentile. “Dr. Patnaik said they were the shortest telomeres he had ever seen,” she recalls. Genetic testing revealed Sutherland had a mutation in the TERT gene, which codes for a protein called telomerase that is responsible for telomere maintenance.
The Mayo team estimates that they have identified a disease-causing variant in a telomere-associated gene in only 40% of patients. In many cases, they have discovered genetic changes that may or may not cause disease — called variants of unknown significance — and have conducted functional studies in the laboratory to categorize the effect of those variants.
Sutherland says she felt relieved when she learned she had a genetic mutation. “You’re always looking for a reason, a cause, an explanation for why this happened. Why did my grandmother, my mother and my sister pass of this horrible disease at such a young age?”
But that sense of relief was quickly replaced by one of foreboding for the mother of five. “I was absolutely, positively scared to death for my children.”
Telomere biology disorders are subject to a troubling type of genetic inheritance known as genetic anticipation. The disorder tends to become more severe and appear at an earlier age as the mutation — and the shortened telomeres — are passed from one generation to the next. As a result, Sutherland worried not only that she had passed the condition on to her children, but also that they might inherit a form of the disease much worse than what she herself has experienced.
“These findings have repercussions for family members,” says Dr. Carmona Porquera. “We can counsel patients: no smoking, no exposures that can cause more injury, be aware of certain symptoms. And we can start treating them earlier with anti-fibrotic drugs, which could slow the progression of the disease.”
Sutherland has been taking anti-fibrotic drugs for the last six years. Though she often feels short of breath, at age 65, she still has the energy to babysit her grandchildren.
Recently, genetic testing revealed no inherited mutations in her family tree.
“Maybe this horrible disease will stop with me,” she says. “That would be my greatest wish.”
This article first published on Discovery’s Edge.
Related articles
[ad_2]
Source link